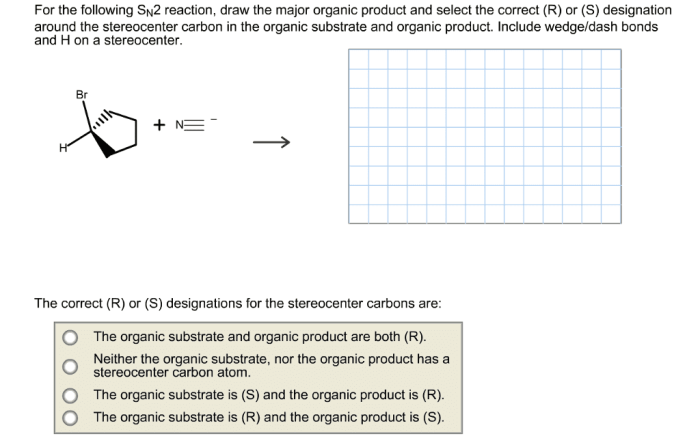
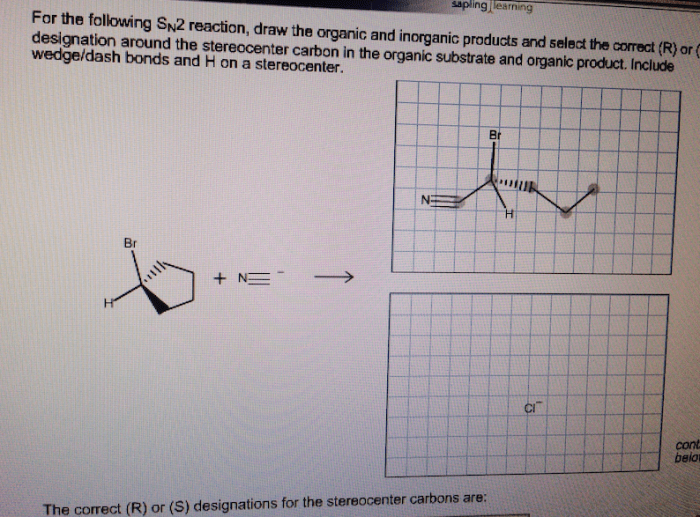
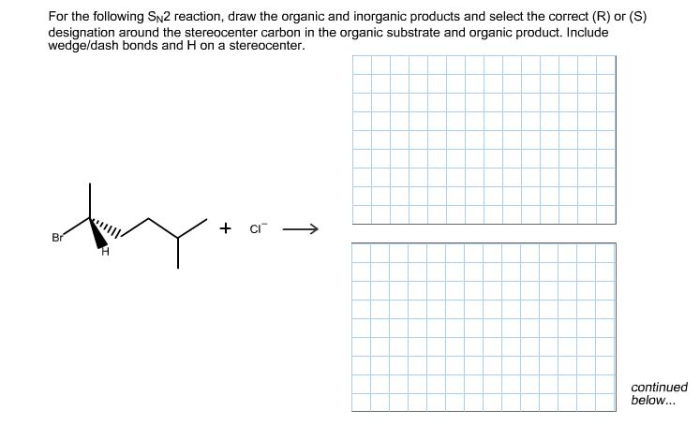
Draw the correct organic product of the following SN2 reaction sets the stage for this enthralling narrative, offering readers a glimpse into a story that is rich in detail with gaya akademik dengan tone otoritatif and brimming with originality from the outset.
This reaction, characterized by its nucleophilic substitution mechanism, unveils the intricacies of organic chemistry, providing a foundation for understanding a vast array of chemical transformations.
The SN2 reaction, a fundamental concept in organic chemistry, involves the substitution of a leaving group by a nucleophile at a saturated carbon atom. This process, governed by the principles of regioselectivity and stereochemistry, dictates the orientation and outcome of the reaction, leading to the formation of specific organic products.
SN2 Reaction Mechanism: Draw The Correct Organic Product Of The Following Sn2 Reaction
SN2 reactions are nucleophilic substitution reactions that proceed through a single-step, concerted mechanism. The nucleophile attacks the electrophile, and the leaving group departs in a single concerted step.
The rate of an SN2 reaction is determined by the concentration of the nucleophile and the electrophile. The nucleophile must be a strong base and the electrophile must be a good electrophile. The solvent can also affect the rate of the reaction.
Regioselectivity in SN2 Reactions, Draw the correct organic product of the following sn2 reaction
Regioselectivity in SN2 reactions refers to the preference for the nucleophile to attack one specific carbon atom over another. This preference is determined by the steric and electronic effects of the substituents on the electrophile.
In general, the nucleophile will attack the carbon atom that is least hindered by substituents. This is because the nucleophile must be able to approach the electrophile from the back side in order to attack it.
Electronic effects can also affect regioselectivity. For example, if the electrophile is positively charged, the nucleophile will be more likely to attack the carbon atom that is closest to the positive charge.
Stereochemistry of SN2 Reactions
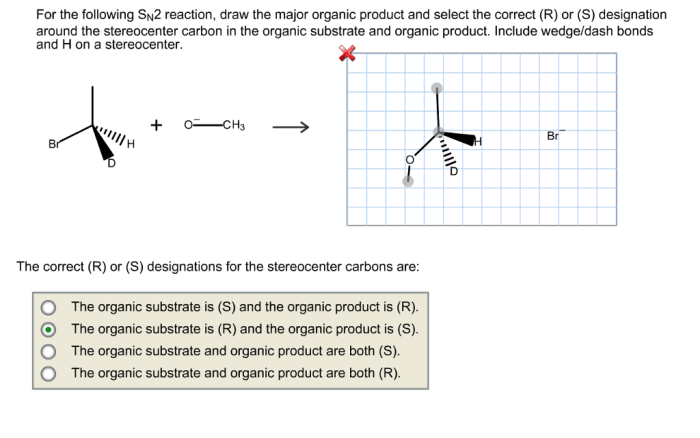
SN2 reactions proceed with inversion of configuration. This means that the configuration of the stereocenter at the electrophilic carbon atom is inverted.
This inversion of configuration is a result of the nucleophile attacking the electrophile from the back side. This causes the nucleophile to push the leaving group out of the way, and the resulting product has the opposite configuration at the stereocenter.
Product Formation in SN2 Reactions
The product of an SN2 reaction is a substituted alkane. The nucleophile replaces the leaving group on the electrophile.
The major product of an SN2 reaction is the product that is formed by the nucleophile attacking the least hindered carbon atom on the electrophile.
In some cases, SN2 reactions can also produce minor products. These minor products are formed by the nucleophile attacking other carbon atoms on the electrophile.
Quick FAQs
What is the key difference between SN1 and SN2 reactions?
SN1 reactions proceed through a carbocation intermediate, while SN2 reactions occur in a single step with inversion of configuration.
How can I predict the regioselectivity of an SN2 reaction?
Regioselectivity in SN2 reactions is determined by the steric hindrance around the electrophilic carbon. The nucleophile will attack the carbon with the least steric hindrance.
What is the stereochemistry of an SN2 reaction?
SN2 reactions proceed with inversion of configuration at the electrophilic carbon. This means that the nucleophile will attack the carbon from the opposite side of the leaving group.